09 Feb Ice Melt
 It’s getting a little later in winter where the freeze-thaw cycle occurs more often. Daytime temperatures, especially starting February 11th, will occasionally reach above freezing during the day and drop below freezing at night. The runoff from the snow melt puts a layer of water down on streets and sidewalks, creating a slick sheet of ice overnight. First and foremost, please be careful on your morning commute. The morning roads may be not be visibly icy—however, you still need to be cautious of ice patches that formed overnight.
It’s getting a little later in winter where the freeze-thaw cycle occurs more often. Daytime temperatures, especially starting February 11th, will occasionally reach above freezing during the day and drop below freezing at night. The runoff from the snow melt puts a layer of water down on streets and sidewalks, creating a slick sheet of ice overnight. First and foremost, please be careful on your morning commute. The morning roads may be not be visibly icy—however, you still need to be cautious of ice patches that formed overnight.
The best way to deal with the freeze-thaw cycle is to use salt or ice melt, and knowing the difference between the two. Salt will usually lower the freezing point of moisture to around 15 degrees. Ice melt typically lowers the freezing point down to around -5 degrees. They both work by lowering the temperature at which water freezes– ice melt just lowers it further. There are also specific types of ice melt that can lower the freezing temperature even further; however they tend to be more expensive. It is important to note that wind chill does not matter for the freeze-thaw cycle—only the actual temperature.
Also, a common misconception about ice melt is that it damages the surfaces it gets put down on (sidewalks, bricks, etc). Chemically speaking, the ice melt interacts with concrete little, if at all. Rather, it is the expediting of the freeze-thaw cycle that weakens the concrete. Using ice melt creates more freeze-thaw cycles than nature would have, however the benefits outweigh the marginal costs in the prevention of falls and accidents.
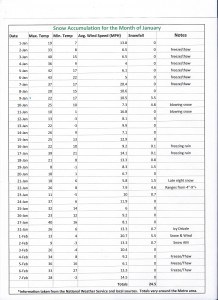
Lastly, this winter has been bitterly cold– however, we have not received as much snow accumulation as last year.


